|
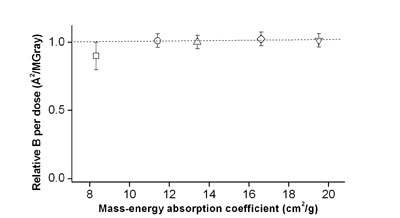 |
Change in relative B
factor per dose at
E=8.8 keV versus mass-energy absorption coefficient for lysozyme
crystals soaked
in iodide solutions. |
| |
|
|
X-ray radiation damage to biological crystal is known to depend on
parameters of the experimental setup (e.g. beam size, shape and energy,
oscillation mode, etc.) as well as on the crystal itself (e.g. its size and
shape, composition, etc.). On one of our experiments, we have measured how
radiation damage at cryogenic temperatures depends on the crystal constituents
and structure of four proteins: lysozyme, catalase, thaumatin, and apoferritin.
We characterize radiation damage as degradation of relative B-factors per
absorbed dose and define a coefficient of sensitivity to absorbed dose that
serves as a robust measure of damage. Our results show that at cryogenic
temperatures, the relative B factor per incident photon fluence increases
linearly with the mass-energy absorption coefficient. The change in relative
B-factor per dose, however, stays roughly independent of the mass-energy
absorption coefficient (given by the crystal composition) and is about the same
for all crystals, with SAD ~ 0.014 Ĺ^2/MGy. These results suggest that cryogenic
radiation sensitivities per absorbed dose are unlikely to show significant
protein-to-protein variations, and that radiation damage may in some cases be
reduced by using salts with lower atomic number constituents. that
serves as a robust measure of damage. Our results show that at cryogenic
temperatures, the relative B factor per incident photon fluence increases
linearly with the mass-energy absorption coefficient. The change in relative
B-factor per dose, however, stays roughly independent of the mass-energy
absorption coefficient (given by the crystal composition) and is about the same
for all crystals, with SAD ~ 0.014 Ĺ^2/MGy. These results suggest that cryogenic
radiation sensitivities per absorbed dose are unlikely to show significant
protein-to-protein variations, and that radiation damage may in some cases be
reduced by using salts with lower atomic number constituents. |
|